423
Views & Citations10
Likes & Shares
ALTERED GLYCOSYLATION IN CANCER
In cancer cells, glycosylation patterns can become aberrant, leading to significant changes in glycan structures on the cell surface and secreted glycoproteins. These alterations in glycosylation have been implicated in several aspects of cancer progression. In tumor growth and proliferation, altered glycosylation patterns can affect the activity of cell surface receptors and signaling molecules, leading to dysregulated growth signals [4]. Additionally, abnormal glycosylation of growth factors and their receptors can promote enhanced mitogenic signaling, thereby contributing to uncontrolled cell proliferation and tumor growth. This enhance the ability of cancer cells to interact with specific tissues, facilitating their migration and invasion to distant sites, a process known as metastasis [5]. In metastasis, glycosylation changes on cell surface proteins can influence cell adhesion, motility, and invasion. In Immune Evasion, Abnormal glycosylation on the surface of cancer cells can help them evade immune surveillance. Certain glycan structures on tumor cells act as "don't eat me" signals, preventing recognition and attack by immune cells, such as natural killer (NK) cells and cytotoxic T cells. This allows cancer cells to escape immune destruction and promote tumor survival [4,5]. Angiogenesis, altered glycosylation can also influence the secretion of pro-angiogenic factors, promoting the formation of new blood vessels (angiogenesis) to supply nutrients and oxygen to growing tumors. This process is crucial for tumor growth and metastasis [6].
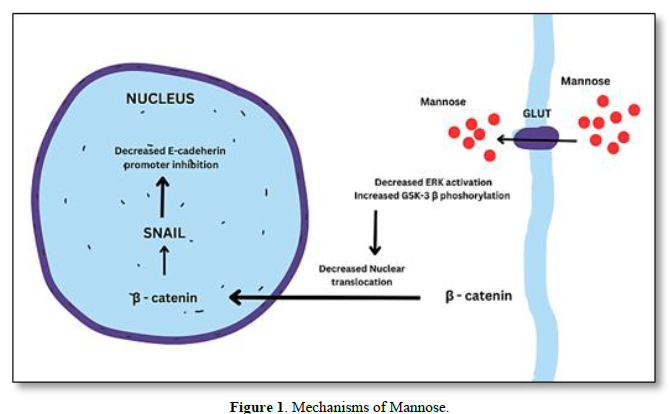
MECHANISMS OF MANNOSE
The uptake of mannose is essential for cancer cells as it serves as a source of energy and can also impact glycosylation processes, which play a crucial role in cancer progression. The uptake of mannose in cancer cells may differ from normal cells due to upregulation of GLUTs, altered glycosylation, increased energy demands, increased mannose receptor expression [7]. As mannose and glucose share some common transporters, such as GLUT1 and GLUT3, cancer cells can utilize these transporters to take up mannose [8]. This uptake can competitively interfere with glucose metabolism and glycosylation pathways in cancer cells. In addition to GLUTs, certain mannose-specific transporters have been identified in various cancer cells. These transporters, such as the mannose transporter SLC5A8, facilitate the uptake of mannose specifically, allowing cancer cells to utilize mannose as an alternative energy source or for glycosylation [9] (Figure 1).
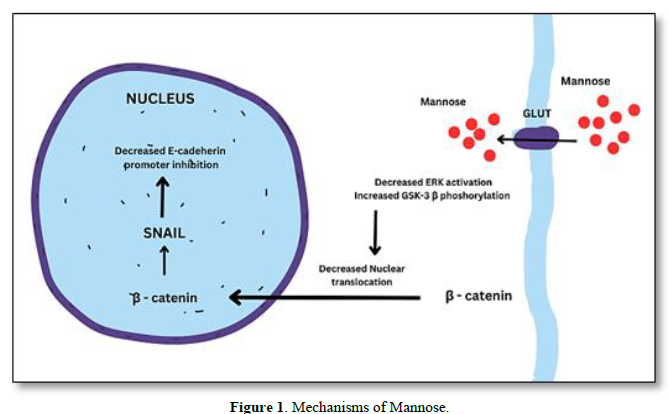
Cancer cells can internalize extracellular molecules, including mannose, through processes like pinocytosis and receptor-mediated endocytosis [10]. In these mechanisms, cancer cells form vesicles to engulf and internalize mannose molecules from the surrounding environment. Abnormal glycosylation patterns on the surface of cancer cells can create unique binding sites for lectins or glycan-binding proteins. These lectins may facilitate the uptake of mannose into cancer cells by binding to specific glycan structures [11] and reduce the ERK activation and then increase the GSK-3 β phosphorylation which will reduce the nuclear translocation of β-catenin in turn down regulates SNAIL expression leads to E- cadherin expression. Thus, overall inhibit the tumor EMT.
Mannose as a Sensitizer to Immune Responses
Mannose can enhance antitumor immune responses, primarily through its effects on immune cell functions by promote dendritic cell maturation and increase T cell activation. Dendritic cells (DC) are critical antigen-presenting cells that play a central role in initiating and regulating immune responses. Immature DCs capture antigens from their surroundings but are less efficient in presenting these antigens to T cells and activating an immune response [12]. Studies have shown that mannose treatment can promote the maturation of dendritic cells. Mannose, when presented to DCs, can activate specific signaling pathways that lead to the upregulation of co-stimulatory molecules such as CD80, CD86 and major histocompatibility complex (MHC) molecules on the DC surface [13]. This, in turn, enhances the presentation of tumor antigens to T cells and primes T cells for an effective antitumor immune response. T cells are a crucial component of the adaptive immune system and play a central role in recognizing and eliminating cancer cells. Mannose has been found to enhance T cell activation, leading to a more robust antitumor immune response [1].
Targeting Specific Cancer Types
The response to mannose treatment in solid tumors and hematological malignancies and depend on several factors, including the specific type of cancer, the expression levels of mannose transporters and receptors, and the tumor microenvironment [10]. The expression levels of glucose transporters (GLUTs) and mannose-specific transporters on the surface of solid tumor cells can influence the uptake of mannose. The tumor microenvironment, including the presence of immune cells, fibroblasts, and blood vessels, can significantly impact the response to mannose treatment [14]. Tumors with an immunosuppressive microenvironment may be less susceptible to the immunomodulatory effects of mannose. In hematological malignancies, such as leukemia and lymphoma, mannose treatment has shown promising potential in preclinical studies. Mannose has been found to enhance the antitumor immune response and induce apoptosis in certain hematological cancer cell lines [15]. The efficacy of mannose treatment in hematological malignancies may be influenced by factors such as the subtype of cancer, the glycosylation patterns of cancer cells, and the interplay between malignant cells and the immune system [14].
Mannose as a Radiosensitizer
Mannose shows promise as a radiosensitizer that enhance the efficacy of radiation therapy in cancer treatment. A radiosensitizer is a substance that makes cancer cells more susceptible to the damaging effects of radiation, thereby increasing the effectiveness of radiotherapy while potentially sparing normal tissues [16,17]. Mannose competes with glucose in glycolysis, a metabolic process that provides cancer cells with energy and helps protect them from radiation-induced damage. By limiting glucose availability and promoting mannose uptake, cancer cells may become more vulnerable to radiation-induced cell death. Mannose treatment has been shown to increase reactive oxygen species (ROS) production in cancer cells [18]. This heightened oxidative stress can lead to DNA damage, amplifying the effects of radiation therapy. Mannose-induced changes in glycosylation may affect the DNA repair capacity of cancer cells, making them more sensitive to radiation-induced DNA damage. Preclinical studies using in vitro and animal models have demonstrated encouraging results regarding the potential of mannose as a radiosensitizer [19]. These studies have shown that mannose treatment before radiation exposure can increase cancer cell death, inhibit tumor growth, and improve survival rates in animal models [18].
CHALLENGES AND LIMITATIONS
Mannose-based face several challenges and limitations that need to be addressed for successful clinical translation and implementation. Cancer cells are known for their ability to develop resistance to various treatments, including targeted therapies. Mannose-based therapies may also face challenges related to the development of resistance mechanisms [20]. Cancer cells might adapt to the altered glycosylation patterns induced by mannose treatment, leading to reduced efficacy over time. The effective concentration of mannose required to induce the desired therapeutic effects while minimizing potential side effects must be carefully determined through preclinical and clinical studies. Additionally, the frequency and duration of treatment should be optimized to achieve the best treatment outcomes. The response to mannose-based therapies may vary depending on the specific characteristics of the cancer cells and tumor microenvironment [21]. Tailoring mannose-based treatments to individual patients or specific cancer subtypes may be necessary to achieve optimal results. While mannose is generally considered safe and well-tolerated, high doses or prolonged exposure may lead to adverse effects. It is essential to carefully monitor patients undergoing mannose-based therapies for any signs of toxicity or unexpected side effects [22]. Understanding potential interactions combination with other cancer treatments, such as radiation therapy, chemotherapy, or immunotherapy and optimizing the sequencing and timing of combination treatments is critical to maximize the therapeutic benefits and minimize potential adverse effects [23].
Biomarkers and Patient Selection
By incorporating biomarkers to predict responsiveness to mannose treatment, personalized cancer therapy approaches can be designed. Patients who are more likely to benefit from mannose-based therapies can be identified, and treatment strategies can be optimized to improve outcomes and patient responses. This approach has the potential to revolutionize cancer treatment by tailoring therapies to individual patients' characteristics and tumor biology. The expression levels of mannose-specific transporters and receptors on cancer cells can indicate the extent of mannose uptake and utilization by the tumor. Higher expression levels of these transporters and receptors may indicate that the cancer cells are more susceptible to mannose treatment [24]. Biomarkers related to specific glycan structures or glycosylation-related enzymes can provide insights into how cancer cells will interact with mannose and how the therapy will impact glycosylation pathways within the tumor. Biomarkers related to immune cell activation, such as T cell activation markers or cytokine profiles, may indicate a patient's potential responsiveness to mannose treatment, especially in combination with immunotherapies [25]. Mannose treatment may influence DNA repair and apoptosis pathways in cancer cells. Biomarkers associated with these processes may help predict how cancer cells will respond to mannose-induced changes in DNA repair and apoptosis [26]. Biomarkers related to glucose metabolism or specific metabolic pathways in cancer cells may provide insights into the impact of mannose treatment on cancer cell energy metabolism. The tumor microenvironment can impact cancer cell response to treatment. Biomarkers related to immune cell infiltration, angiogenesis, or stromal components may help identify patients whose tumors are more likely to respond to mannose-based therapies [27]. As a radiosensitizer, mannose can enhance the efficacy of radiation therapy. Biomarkers related to cellular responses to radiation, such as DNA damage repair or oxidative stress markers, may indicate whether a patient could benefit from combining mannose with radiation therapy.
Toxicity in Normal Cells
Supplement or therapeutic agent, mannose can have potential toxicity if consumed or administered in excessive amounts or under certain conditions. Studies assessing the impact of mannose supplementation on non-cancerous cells and tissues have provided insights into its safety profile. Animal Studies: Several animal studies have investigated the safety of mannose supplementation on non-cancerous tissues. In these studies, animals were given mannose orally or through intravenous administration, and various parameters were assessed, including general health, organ function, and histological examinations [28]. The majority of these studies have reported no significant toxicity or adverse effects on normal tissues at the tested doses. In a systematic review and meta-analysis of randomized controlled trials, the safety of mannose supplementation was assessed in healthy volunteers. The review concluded that mannose was generally safe, with no significant differences in adverse events between mannose supplementation and placebo groups. Studies have suggested that high-dose mannose supplementation might potentially impact renal function. However, the clinical relevance of this finding remains uncertain, and the significance may depend on the specific context and individual patient characteristics. In some cases, high doses of mannose supplementation have been associated with gastrointestinal disturbances, such as diarrhea and bloating [29]. These effects are typically transient and resolve once the mannose supplementation is discontinued or reduced. Mannose is structurally similar to glucose, and high doses of mannose can affect blood glucose levels. In individuals with diabetes or impaired glucose metabolism, close monitoring of blood glucose levels is necessary when taking mannose supplements to prevent any potential adverse effects [30].
CONCLUSION
Mannose holds significant promise as a potential cancer treatment due to its crucial role in cellular processes, especially glycosylation. It impacts various aspects of cancer progression, including tumor growth, metastasis, immune evasion, and angiogenesis. Mannose-based therapies have shown potential as immunomodulatory agents, radio sensitizers, and can enhance antitumor immune responses. Challenges such as potential resistance mechanisms, optimal dosing, and safety considerations need to be addressed. Additionally, identifying biomarkers to predict patient responsiveness and understanding the complex interplay between mannose and cancer cells' characteristics and microenvironment are crucial for successful clinical implementation. Further research, including well-designed clinical trials and translational studies, is necessary to fully explore the efficacy, safety, and potential combination therapies involving mannose. This will aid in understanding the optimal use of mannose-based treatments and its potential role in personalized cancer therapy approaches. In conclusion, mannose offers promising potential as a cancer treatment, and its multifaceted mechanisms of action make it an intriguing therapeutic approach. However, more comprehensive research is needed to harness its full potential and to establish mannose-based therapies as safe, effective, and transformative options for cancer patients.
- Sharma V, Ichikawa M, Freeze HH (2014) Mannose metabolism: More than meets the eye. Biochem Biophys Res Commun 453(2): 220-228.
- Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15(6): 346-366.
- Lin B, Qing X, Liao J, Zhuo K (2020) Role of Protein Glycosylation in Host-Pathogen Interaction. Cells 9(4): 1022.
- Thomas D, Rathinavel AK, Radhakrishnan P (2021) Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim Biophys Acta Rev Cancer 1875(1): 188464.
- Khosrowabadi E, Wenta T, Keskitalo S, Manninen A, Kellokumpu S (2022) Altered glycosylation of several metastasis-associated glycoproteins with terminal GalNAc defines the highly invasive cancer cell phenotype. Oncotarget 13: 73-89.
- Sun R, Kim AMJ, Lim SO (2021) Glycosylation of Immune Receptors in Cancer. Cells 10(5): 1100.
- Pliszka M, Szablewski L (2021) Glucose Transporters as a Target for Anticancer Therapy. Cancers (Basel) 13(16): 4184.
- Pragallapati S, Manyam R (2019) Glucose transporter 1 in health and disease. J Oral Maxillofac Pathol 23(3): 443-449.
- Panneerselvam K, Freeze HH (1996) Mannose enters mammalian cells using a specific transporter that is insensitive to glucose. J Biol Chem 271(16): 9417-9421.
- Nan F, Sun Y, Liang H, Zhou J, Xiao M, et al. (2022) Mannose: A Sweet Option in the Treatment of Cancer and Inflammation. Front Pharmacol 13: 877543.
- Mazlan MA, Isa MLM, Ibrahim M (2020) A high mannose concentration is well tolerated by colorectal adenocarcinoma and melanoma cells but toxic to normal human gingival fibroblast: An in vitro Egypt J Med Hum Genet 21: 64.
- Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, et al. (2023) Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol 20 (5): 432-447.
- Schmidt SV, Nino-Castro AC, Schultze JL (2012) Regulatory dendritic cells: There is more than just immune activation. Front Immunol 3: 274.
- Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, et al. (2020) Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci 7: 193.
- Ying Y, Hao W (2023) Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front Immunol 14: 1147641.
- Zhang Y, Li Q, Huang Z, Li B, Nice EC, et al. (2022) Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies. Cancers (Basel) 14(19): 4568.
- Gong L, Zhang Y, Liu C, Zhang M, Han S (2021) Application of Radiosensitizers in Cancer Radiotherapy. Int J Nanomedicine 16: 1083-1102.
- Kim W, Lee S, Seo D, Kim D, Kim K, et al. (2019) Cellular Stress Responses in Radiotherapy. Cells 8(9): 1105.
- Nong S, Han X, Xiang Y, Qian Y, Wei Y, et al. (2023) Metabolic reprogramming in cancer: Mechanisms and therapeutics. Med Comm 4: e218.
- Zhu Q, Huang Y, Zhu X, Peng L, Wang H, et al. (2023) Mannose-coated superparamagnetic iron oxide nanozyme for preventing postoperative cognitive dysfunction. Mater Today Bio 19: 100568.
- Xiao Y, Yu D (2021) Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 221: 107753.
- Schirrmacher V (2019) From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol 54(2): 407-419.
- Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4): 237-251.
- Zhang R, Yang Y, Dong W, Lin M, He J, et al. (2022) D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci USA 119(8): e2114851119.
- Ščupáková K, Adelaja OT, Balluff B, Ayyappan V, Tressler CM, et al. (2021) Clinical importance of high-mannose, fucosylated, and complex N-glycans in breast cancer metastasis. JCI Insight 6(24): e146945.
- Gonzalez PS, O'Prey J, Cardaci S, Barthet VJA, Sakamaki JI, et al. (2018) Mannose impairs tumors growth and enhances chemotherapy. Nature 563(7733): 719-723.
- Greco B, Malacarne V, De Girardi F, Scotti GM, Manfredi F, et al. (2022) Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci Transl Med 14(628): eabg3072.
- Kulcsár G (2000) Synergistic potentiating effect of D (+)-mannose, orotic, and hippuric acid sodium salt on selective toxicity of a mixture of 13 substances of the circulatory system in culture for various tumor cell lines. Cancer Detect Prev 24(5): 485-495.
- Dong L, Xie J, Wang Y, Jiang H, Chen K, et al. (2022) Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun 13(1): 4804.
- Hernández D, De la Fuente M. (1989) Mannose toxicity in Ehrlich ascites tumor cells. Biochem Cell Biol 67(6): 311-314.